Cutting-Edge Research
in Synthesis and Development of Analytical Materials:
-
Molecular Imprinting Science
-
Responsive Hydrogels
-
Synthesis & Application of Engineered Surrogate Soils

CURRENT RESEARCH


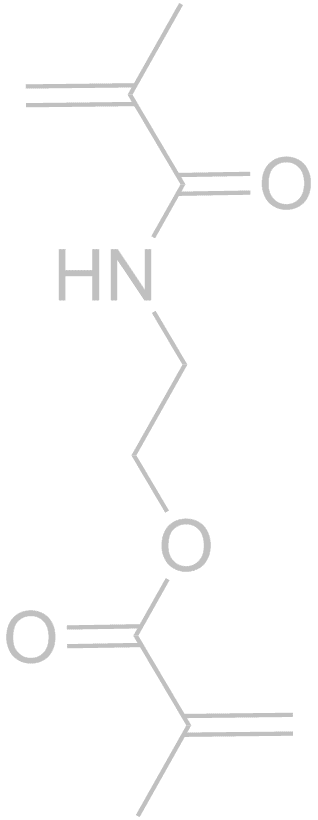
Molecularly Imprinted Polymers (MIPs) are formed using a template molecule complexed to interactive monomers and polymerized. Subsequent removal of the template leaves a specific binding cavity that selectively rebinds the template versus any other molecule. There are now tens of thousands of publications on the subject, but few introduce innovative concepts like those developed in our group.With respect to more traditional MIP strategies, our group was the first to rigorously show that shape selectivity is an important component of molecular recognition in MIPs. Through a series of papers we showed that shape selectivity is most effective when there is 1 non-covalent interaction of the template with the MIP, and subordinate to functional group pre-organization for MIPs with 3 or more non-covalent interactions (1. Anal. Chim. Acta, 2004, 504, 23-30. 2. Anal Chim Acta, 2007, 591, 7-16). In another “discovery-based” MIP materials project, the use of just one monomer (Scheme above) for MIPs, referred to as OMNiMIPs (for one monomer molecularly imprinted polymers) has revolutionized the field because it has solved many problems of copolymer formulations and is significantly easier to carry out in practice (J. Am. Chem. Soc. 2004, 126, 7827-7833). We have an ongoing effort toward design and synthesis of new crosslinkers that can improve the OMNiMIP method (Anal. Bioanal. Chem., 2007, 389, 433-440). Recently we have reported the first example of using chiral chromatography to determine the stereochemistry of a single enantiomer analyte (Organic Letters, 2014, 16, 1402–1405). MIPs are not required for this method, but do provide a straightforward method of obtaining enantiomeric stationary phases.

Our group is currently funded for the design and synthesis of engineered soil surrogates, in collaboration with the Cook group at LSU. The ultimate goal for the design of engineered soil surrogates is to mimic the 3-tiered hierarchy of general soil architecture that includes an aliphatic “lipid-like” layer, a “lignin-like” aromatic layer, and a polar layer mimicking water-interactive cellulosic and carboxylate zones. The synthesis of the engineered soil particles is modular, allowing substitution of different chemistries in each zone for evaluation and comparison to real-world soils. For example, replacing the oligomeric carboxylic acid group for an oligosaccharide group may provide better soil pH while maintaining hydrophilicity.


An innovative type of MIPs recently reported by our group are aptamer-based hydrogels with specific response to target protein and virus biomarkers (Scheme 2. Journal of the American Chemical Society, 2013, 135, 6977-6984. Scheme 3. Angewandte Chemie International Edition, 2014, 53, 2095–2098). A remarkable aspect of these super-aptamer hydrogels is that volume shrinking is visible to the naked eye down to femtomolar concentrations of protein. This extraordinary macromolecular amplification is attributed to a complex interplay between protein-aptamer supramolecular crosslinks and the consequential reduction of excluded volume in the hydrogel. Specific recognition is even maintained in biological matrices such as urine and tears. To increase accuracy of naked-eye evaluation of the shrinking response, we have also developed diffraction grating imprinted hydrogels that have also been bioimprinted with a virus. Changes in diffraction distances of transmitted laser light gives a sensitive measure of the virus binding.